Prosthetically Guided Implant Placement: Customized Bone Regeneration Techniques with Custom-Made Titanium Grids Yxoss CBR
Машинний переклад
Оригінальна стаття написана мовою IT (посилання для прочитання).
The placement of the dental implant is an effective treatment for the replacement of lost teeth, with high long-term implant survival rates.
The fundamental factor for the success of implantology is the availability of adequate bone structure. For dental implants to be successful, the bone must be sufficient both quantitatively (in terms of height and/or width) and qualitatively (ample vascularization).
The lack of adequate bone volume is a common deterrent to the placement of dental implants and also a cause of implant failure, both in the healing/osseointegration phase and in the restoration phase.
An adequate bone volume therefore represents an important prerequisite for a predictable long-term prognosis in implant dentistry and for a functionally and aesthetically correct rehabilitation. The residual bone volume is often insufficient to allow for the insertion of dental implants according to the criteria of prosthetic implantology, as reported by recent guidelines. Therefore, the restoration of an adequate amount of bone and an adequate contour of the alveolar ridge should be considered in any treatment plan, to allow for prosthetically guided implant placement.
Barrier membranes have been used in dental implantology since the late 1980s in the guided bone regeneration (GBR) procedure. This barrier aims to prevent the inward growth of non-osteogenic connective tissue cells into the bone defect and to create a space where a blood clot can organize, leading to the formation of new bone. The ideal characteristics of the membrane include: biocompatibility, easy manipulation, integration with surrounding tissues, space maintenance, and wound stabilization.
The management of soft tissues is one of the key points of GBR. Closure of the wound by first intention without tension is mandatory when performing any bone augmentation procedure. Otherwise, early wound dehiscence will occur.
Guided bone regeneration can be performed in two ways:
- Two-stage procedure: GBR is performed first, and after a period of 4-9 months, the dental implant can be inserted into the correct 3D position in the healing bone;
- One-stage procedure: the dental implant is inserted simultaneously with bone regeneration; this procedure is feasible when the dental implant can be placed in the correct 3D position with sufficient primary stability and with the closure of the wound by first intention without tension.
The use of titanium mesh in guided bone regeneration is a common procedure for horizontal and vertical ridge augmentation. Clinical and histological analysis has revealed an increase in the ridge and formation of new bone after the application of a titanium mesh along with deproteinized bovine bone mineral (DBBM) and autologous bone. A greater vertical gain of the ridge can be achieved using titanium meshes. The main disadvantage of traditional titanium meshes is the manual 3-D intraoperative cutting, which is time-consuming to achieve the desired final shape.
CAD/CAM technology can now be used to overcome these disadvantages. By using computed tomography (CT) of the individual patient or cone beam computed tomography (CBCT), the volume of augmentation needed for the defects can be estimated preoperatively, allowing for the creation of a defect-specific mesh. The custom-made titanium meshes Yxoss CBR are made using the patient's DICOM data, in order to avoid the intraoperative adaptation of traditional titanium meshes. The application of a custom-made mesh reduces the duration of the procedure and can thus lower the overall treatment costs. The most common complication of titanium meshes is flap dehiscence. Sagheb et al. published a study on the use of custom-made titanium meshes Yxoss CBR for alveolar ridge augmentation and demonstrated that there is no negative impact of any dehiscences that occurred on the treatment outcome.
Seiler et al reported the treatment of 115 alveolar ridge defects, reconstructed with Yxoss CBR grids in association with autologous bone particles and DBBM, with or without the coverage of a native collagen membrane. The study demonstrated healing without complications in 82 defects. Infection of the surgical area was documented in 11 cases, of which 10 were resolved with medication. Dehiscence was reported in 26 defects (exposure rate of 22.6%). Premature removal of exposed grids was not necessary. All cases showed sufficient bone regeneration for implant placement with preoperative 3-D planning. The volume grafted in defects with dehiscence did not differ from that in sites without dehiscence. Statistical analysis revealed no correlation between dehiscence and demographic or surgical parameters, but a trend towards a higher prevalence of dehiscence with increasing mesio-distal width of the defect. They concluded that the combination of a custom-made titanium grid Yxoss CBR with guided bone regeneration is a reliable grafting technique with low sensitivity to dehiscence.
Recently, the study by Chiapasco et al. confirmed the result on the exposure rate. Out of a total of 53 sites treated with custom-made titanium grids Yxoss CBR and a mix of autologous bone and DBBM, 11 showed grid exposures (exposure rate of 20.75%): in 8 of them, the integration of the graft occurred without issues, while in 3 there was a partial bone loss. In the sites that experienced partial bone loss, it was still possible to deliver the planned prosthetic restorations. The work also demonstrated a high bone gain (the average vertical and horizontal bone increase was 4.78±1.88 mm and 6.35±2.10 mm, respectively) and an implant survival rate of 100%22. Dellavia et al. performed a histological analysis of the regenerated bone with Yxoss CBR grids in association with a mixture of autologous bone and DBBM, at 9 months post-healing. In all sites, the newly formed tissue was found to be highly mineralized, well-organized, and composed of 35.88% new lamellar bone, 16.42% woven bone, 10.88% osteoid matrix, 14.10% graft remnants, and 22.72% marrow spaces. Blood vessels constituted 4% of the tissue. These data demonstrated that regeneration with Yxoss CBR grids, in association with autologous bone and DBBM, allows for adequate vascularization and vitality of the regenerated bone.
Furthermore, even in the case of GBR for the treatment of extensive defects using a Yxoss CBR grid in combination with a mix of autologous bone and DBBM, in a ratio favoring the latter, the quality of the newly formed bone is optimal.
In the case report by Garocchio, published in Implants, the result of the histological examination performed nine months after the intervention with custom-made titanium Yxoss CBR grids showed that the bone presents a normal morphology, characterized by newly formed bone trabeculae that delimit large medullary spaces rich in vessels. No osteoclastic cells were identified, indicating that remodeling is minimal and that the bone is in an advanced stage of organization and maturation.
Clinical Case
The patient presents to our observation complaining of aesthetic discomfort and frequent abscess episodes. In area 1.1, we find the presence of an implant-supported restoration and a continuous solution at the level of the vestibular marginal gingiva. Radiographic examination reveals significant bone loss related to implant mispositioning. The excessive depth of implant insertion and incorrect management of the transmucosal pathway have caused aesthetic and functional damage (Figs. 1-3).
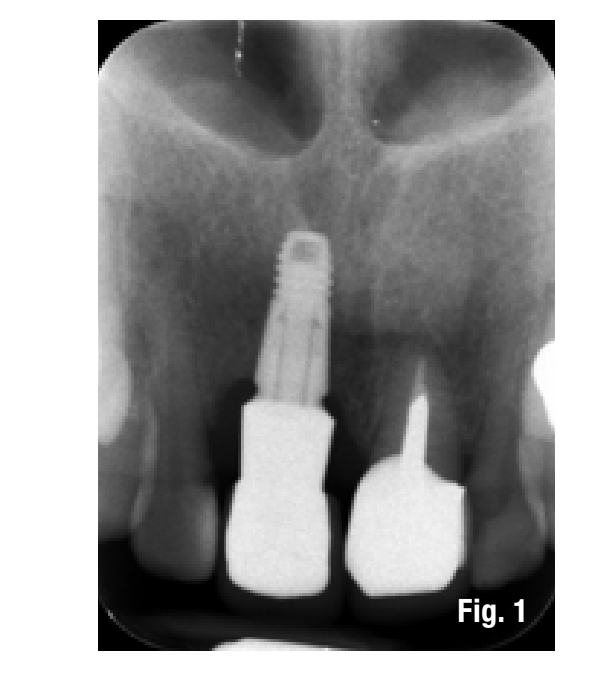
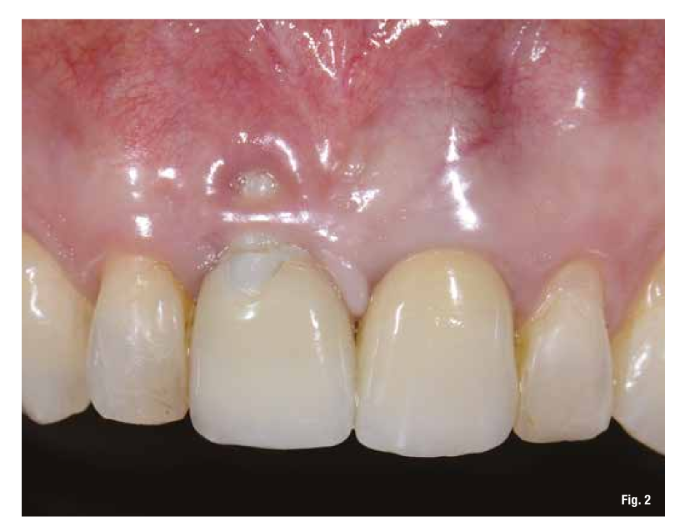
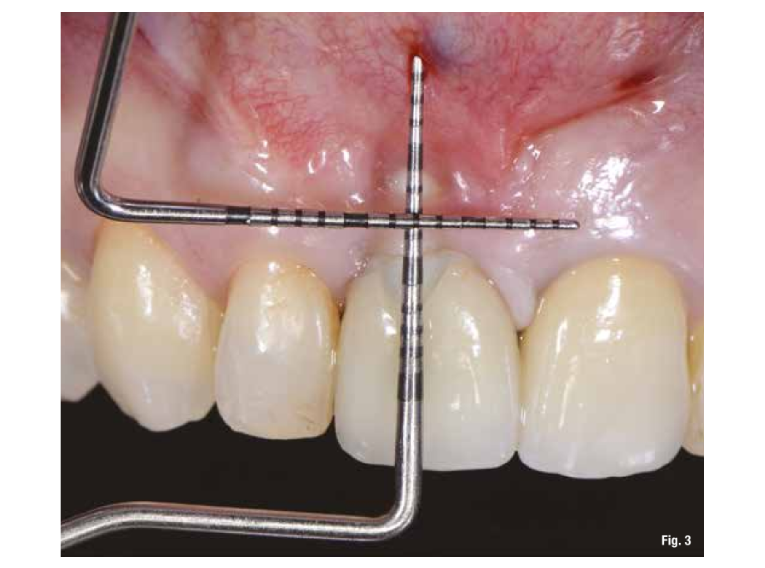
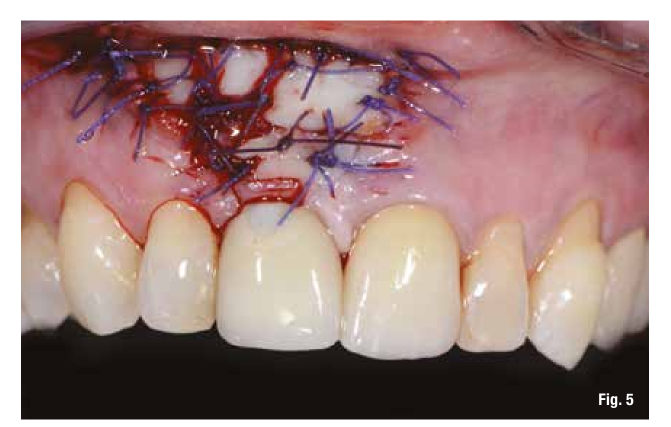
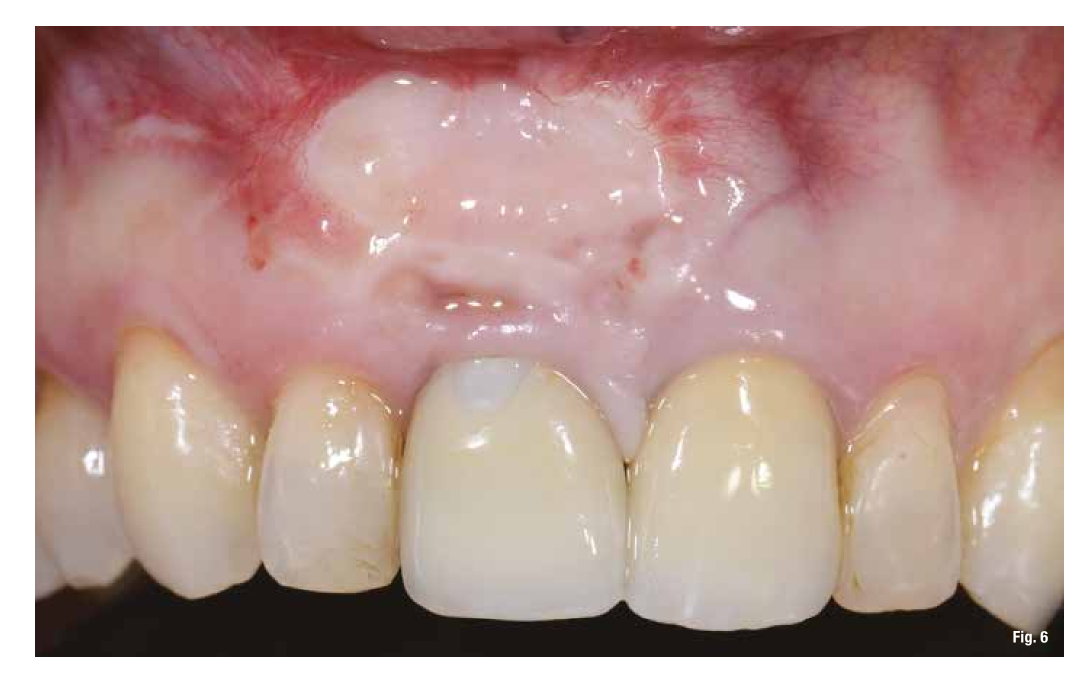
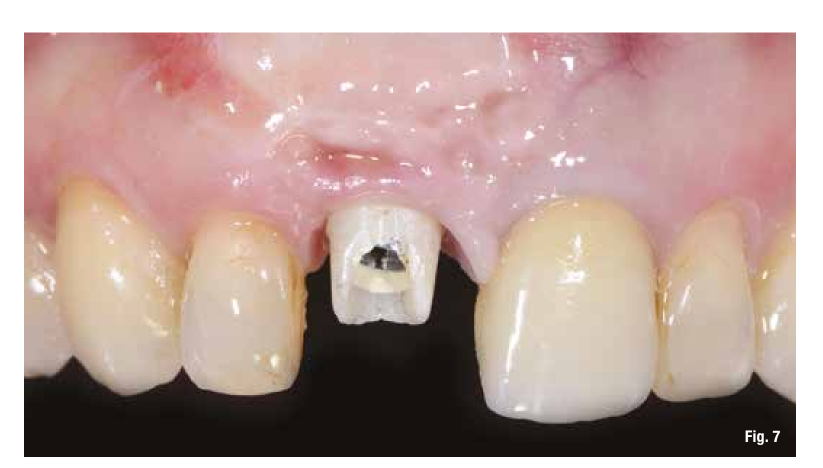
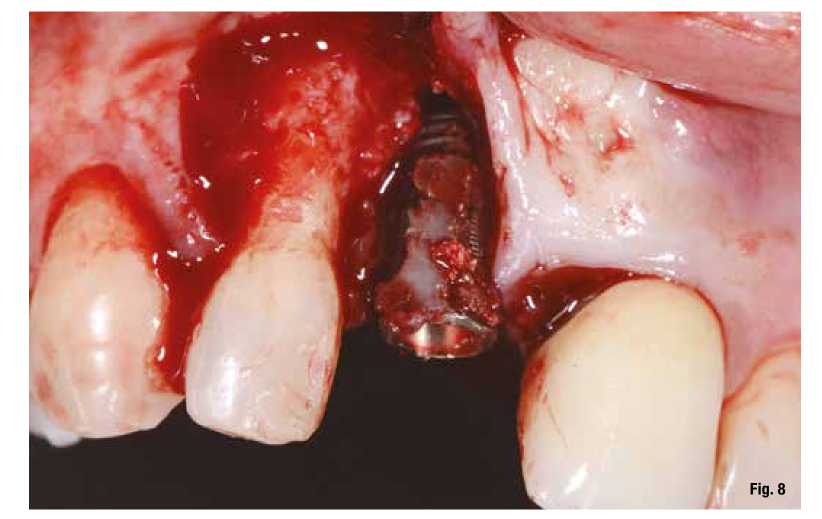
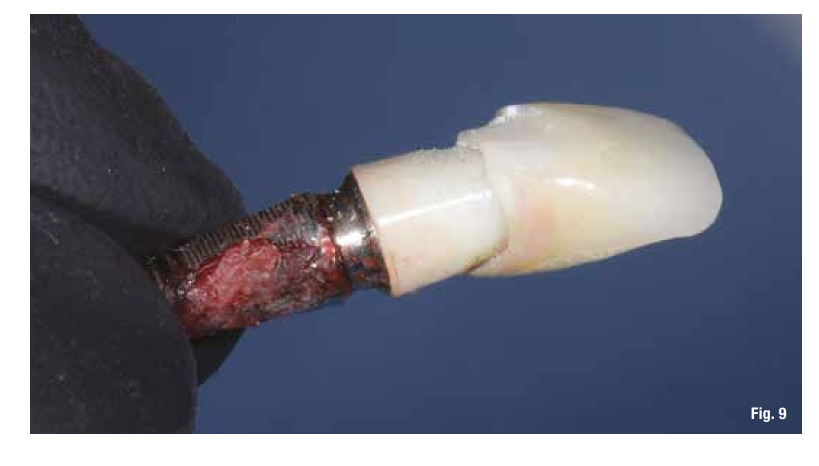
The proposed treatment plan requires the removal of the implant and the management of the residual defect using GBR technique. Regardless of the chosen technique, for the correct management of regenerative procedures, we need a quality and quantity of soft tissues sufficient to support the coverage of the defect. Upon analyzing the site to be treated, we highlight the total absence of attached gingiva, and for this reason, we plan for a tissue augmentation using a free gingival graft (Figs. 4, 5). For the maturation of the soft tissues, we waited three months before proceeding with the removal of the implant (Fig. 6). In these cases, the removal of the implant is complicated and is associated with further bone loss. Once the implant is removed, we can better appreciate the incorrect prosthetic implant treatment (Figs. 7-9). We wait about three months and in the meantime, we begin to design the regenerative technique. We do this by analyzing a CBCT and a guided surgery software that allows us to understand the extent of the necessary bone regeneration. For some time now, to make the regenerative technique simplified and predictable, we have been using titanium grids that are produced based on cone beam images, wax-ups, and the indications we provide to the manufacturer.
The design of the bone reconstruction was therefore done in a Prosthetically Guided manner, positioning the virtual implant and then assessing the extent of the necessary regeneration.
Once all the necessary information from the bone data (cone beam) and the prosthetic planning (STL of the wax-up, the tissues, and the initial situation) is obtained, we send everything to the company (ReOss, Filderstadt), which, after the design, provides us with the design of the Yxoss CBR grid that can be viewed in three dimensions and possibly modified at the discretion of the professional. Suggested modifications are made, and the final validation of the project for the realization of the grid is executed (Fig. 10). With Customized Bone Regeneration (CBR), the goal of Digitally Guided Prosthetic Regeneration is thus achieved.

The grids are produced through three-dimensional printing and their fit to the defect is always very accurate, allowing us to focus on the other phases of the surgery.
The management of the intervention becomes very simple; our attention must be directed to a correct design of the flap, because with a defect-specific grid available, we do not need to waste time cutting membranes or the classic titanium grids. The primary incision of the flap is different from what we usually perform for GBR procedures with membranes. The first incision is made at the crest or, in some cases, even more vestibular towards the defect. This way, we leave more palatal tissue to cover the grid, and the vestibular flap is passivated through periosteal and muscular release maneuvers.
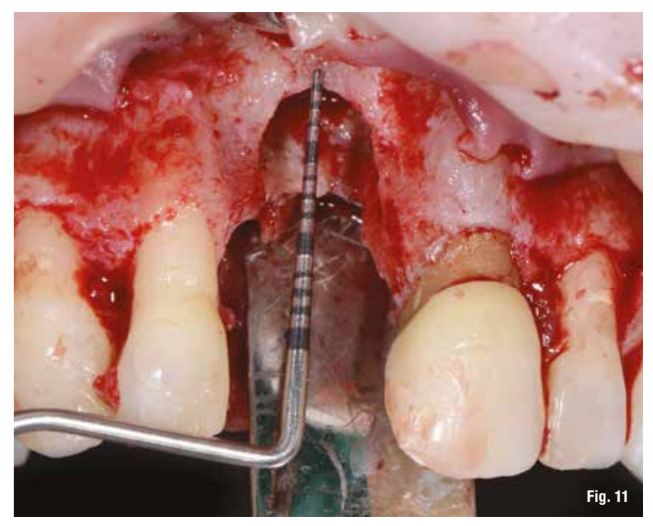
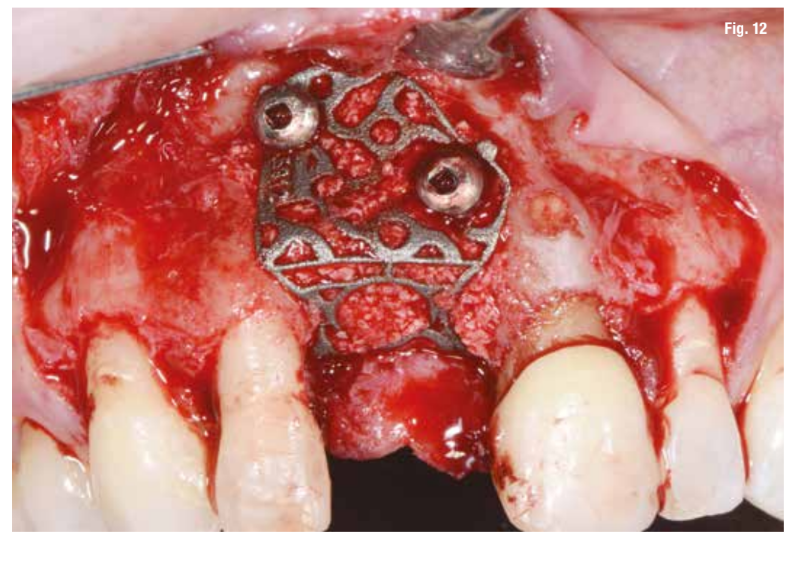
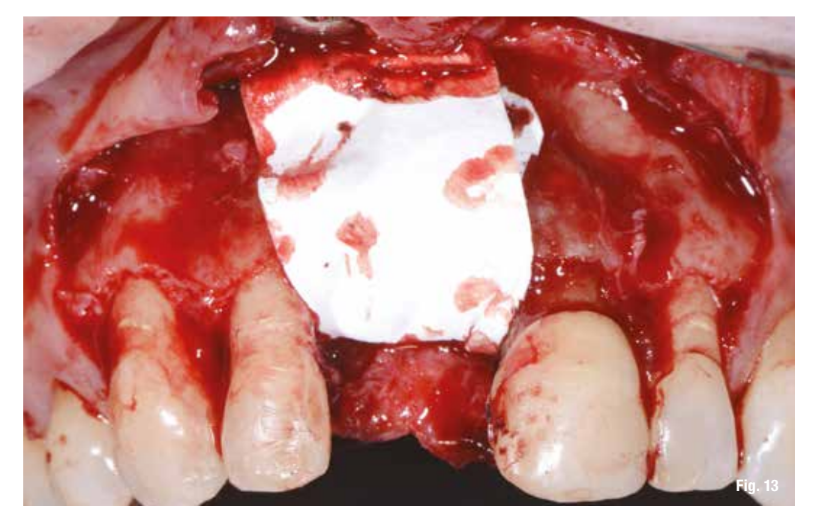
After performing the debridement of the defect (Fig. 11), we first test the fit of the grid and then fill it with a mixture of autologous bone, collected with Safe Scraper, and 50% deproteinized bovine bone mineral (Geistlich Bio-Oss, Geistlich Pharma AG). The grid is then secured with mini-screws (Fig. 12) and covered with a resorbable collagen membrane (Geistlich Bio-Gide, Geistlich Pharma AG) to enhance tissue healing and to prevent the migration of soft tissue into the graft (Fig. 13).
The flap, after being released from the periosteum and muscle planes, is repositioned without tension. The sutures are made with horizontal mattress sutures combined with interrupted sutures on the superficial part of the flap, achieving primary intention wound closure (Fig. 14).
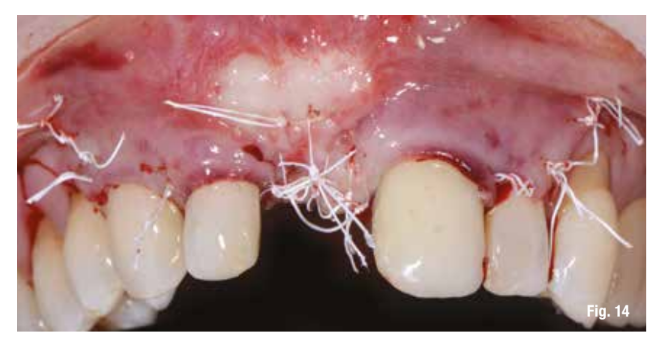
Postoperative control is fundamental (Fig. 15). The sutures are removed after 15 days and the check-up after one month shows good healing of the tissues.
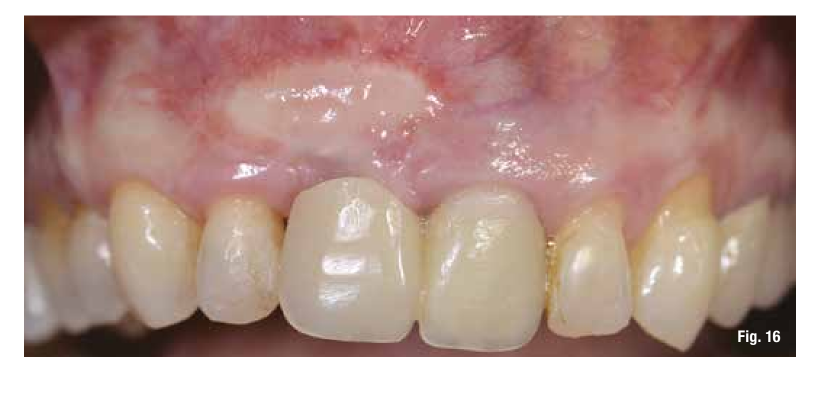
Before proceeding with implant placement, we make a modification of the graft color through a simple surgical technique (Fig. 16).
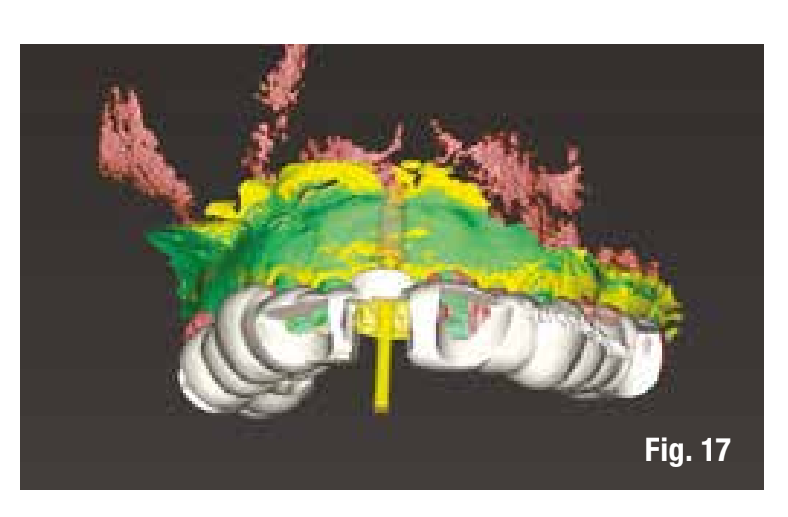
To reduce the number of interventions, we design the implant placement to be prosthetically guided through the use of computer-guided surgery to be performed after the removal of the grid. The images in the software allow us to insert the implant into the regenerated bone in a prosthetically guided manner (Figs. 17, 18).
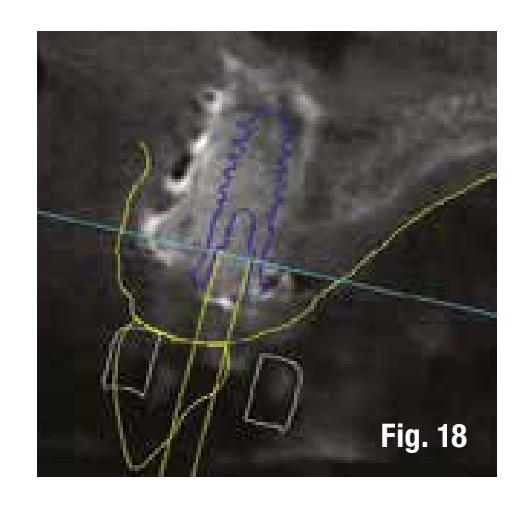
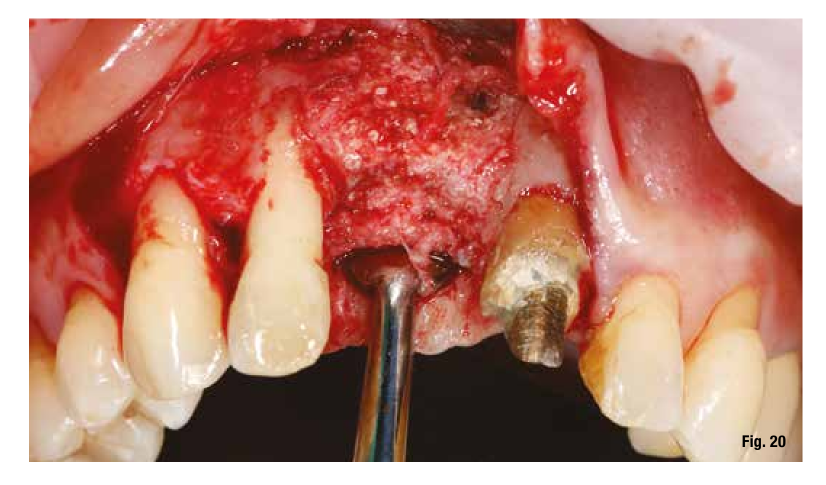
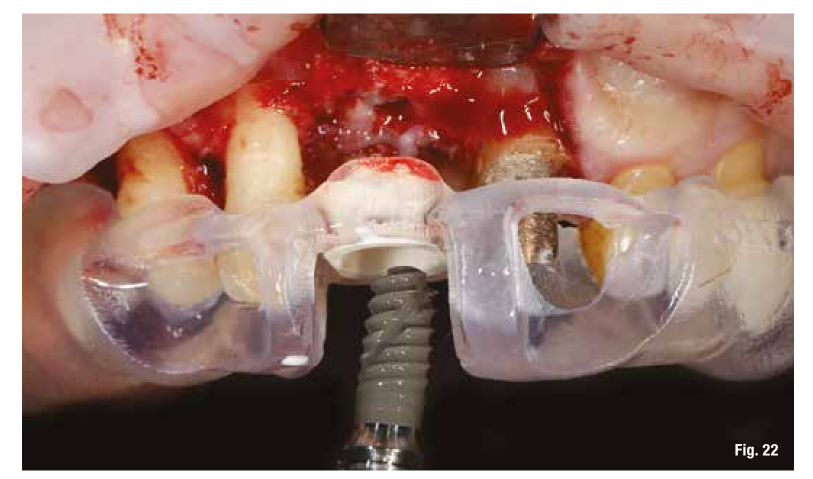
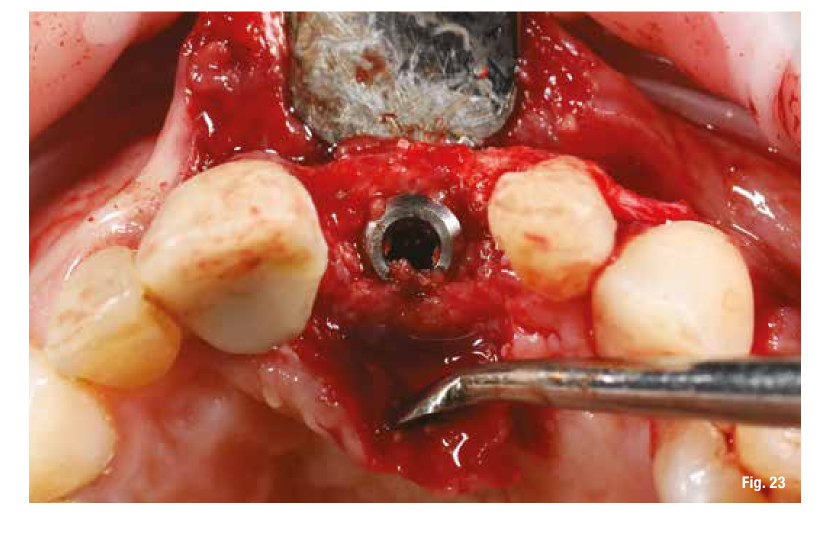
The removal of the grid, which occurs after 6 months, in these cases is very simple and is performed by creating a lever effect at the cutting points provided in the grid that separate the vestibular part from the palatal part (Fig. 19). Once the two portions of the grid are removed, we check the extent and quality of the regeneration (Fig. 20) before proceeding with the preparation of the implant bed using the surgical guide (Figs. 21, 22). In sites with high aesthetic value and with an implant between two natural elements, the depth of implant insertion is fundamental and, if planned before surgery, allows us to be more predictable (Fig. 23).


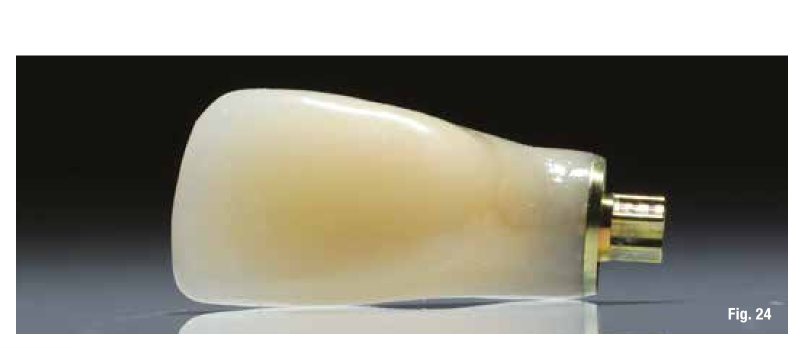
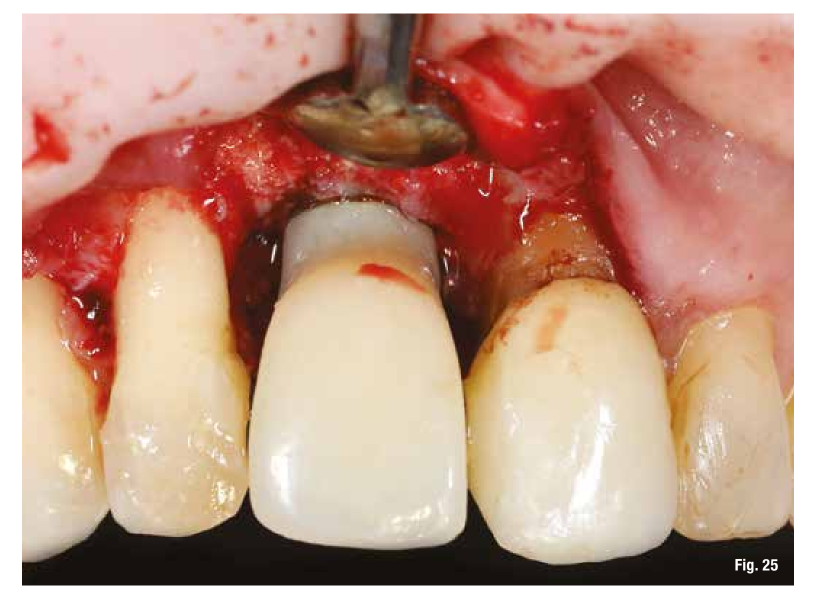
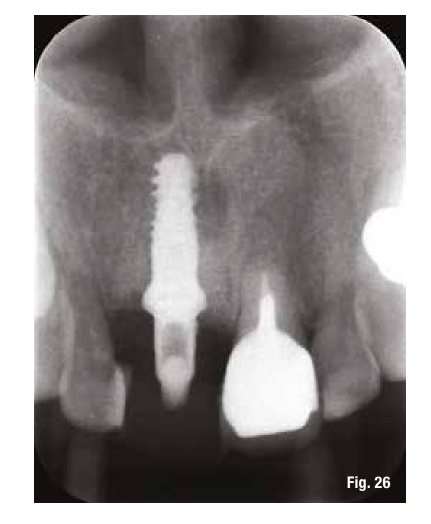
The use of the surgical guide ensures the correct insertion depth, and therefore we choose an implant with a performance spiral morphology (TLX Straumann). Once the implant is inserted and the insertion torque is verified, we also place the immediate restoration to condition the peri-implant tissues right away (Figs. 24-26).
Conclusions
Guided bone regeneration is a well-established and well-documented procedure for correcting horizontal and/or vertical bone deficiency. This technique has a very high success rate. The use of GBR techniques with custom-made titanium grids Yxoss CBR has proven to be a valid surgical solution, especially for vertical and combined defects. The management of soft tissues for adequate coverage remains one of the most critical steps of this technique. However, exposure of the grid does not result in complete loss of the graft. The clinical case presented shows that the Yxoss CBR grid can ensure excellent bone regeneration, with the advantage of speeding up and optimizing the surgical procedure. These premises allow for prosthetically guided implant placement, ensuring the foundations for functionally and aesthetically adequate implant-prosthetic rehabilitation.
Santo Garocchio, Filippo Tomarelli, Maurizio De Francesco, Giuseppe Marano
Bibliography:
- Moraschini V., Poubel LA., Ferreira VF., Barboza ES.. Evaluation of survival and success rates of dental implants reported in longitudinal studies with a follow-up period of at least 10 years: a systematic review. Int J Oral Maxillofac Surg. 2015;44(3):377–88. PubMed PMID:25467739.
- Al-Nawas B., Kammerer PW., Morbach T., Ladwein C., Wegener J., Wagner W.. Ten-year retrospective follow-up study of the TiOblast dental implant. Clin Implant Dent Relat Res. 2012;14(1):127–34. PubMed PMID: 20156231.
- Schiegnitz E., Al-Nawas B., Tegner A., Sagheb K., Berres M., Kammerer PW., et al. Clinical and radiological long-term outcome of a tapered implant system with special emphasis on the influence of augmentation procedures. Clin Implant Dent Relat Res. 2016;18(4):810–20. PubMed PMID: 25810365.
- Jung RE., Fenner N, Hammerle CHF., Zitzmann NU.. Long-term outcome of implants placed with guided bone regeneration (GBR) using resorbable and non-resorbable membranes after 12–14 years. Clin. Oral Impl. Res. 24, 2013, 1065–1073.
- Setiawan Budihardja A., Mücke T.. “Bone Management in Dental Implantology”.
- Cucchi et al. Clinical and volumetric outcomes after vertical ridge augmentation using computer-aided-design/computer-aided manufacturing (CAD/CAM) customized titanium meshes: a pilot study. BMC Oral Health (2020) 20:219.
- De Santis D., Cucchi A., Rigoni G., Longhi C.. Short implants with oxidized surface in posterior areas of atrophic jaws: 3- to 5-year results of a multicenter study. Clin Implant Dent Relat Res. 2015;17(3):442–52.
- Malchiodi L., Ghensi P., Cucchi A., Pieroni S., Bertossi D.. Peri-implant conditions around sintered porous-surfaced (SPS) implants. A 36-month prospective cohort study. Clin Oral Implants Res.2015;26(2):212–9.
- Rocchietta I., Ferrantino L., Simion M.. Vertical ridge augmentation in the esthetic zone. Periodontol. 2018;77(1):241–55.
- Cucchi A., Chierico A., Fontana F., Mazzocco F., Cinquegrana C., Belleggia F., Rossetti P., Soardi CM., Todisco M., Luongo R., Signorini L., Ronda M., Pistilli R. Statements and recommendations for guided bone regeneration: consensus report of the guided bone regeneration symposium held in Bologna, October 15 to 16, 2016. Implant Dent. 2019;28(4):388–99.
- Buser D., Bornstein MM., Weber HP., Grutter L., Schmid B., Belser UC. Early implant placement with simultaneous guided bone regeneration following single tooth extraction in the esthetic zone. A cross sectional, retrospective study in 45 subjects with 2 to 4 year follow up. J Periodontol. 2008;79:1773–81.
- Hammerle CH., Lang NP.. Single stage surgery combining transmucosal implant placement with guided bone regeneration and bioresorbable materials. Clin Oral Implants Res. 2001;12:9–18.
- Hurzeler MB., Strub JR.. Guided bone regeneration around exposed implants. A new bioresorbable device and bioresorbable membrane pins. Pract Periodontics Aesthet Dent. 1995;7:37–47.
- Buser D., Dahlin C.. Bone grafts and bone substitute materials. In: Buser D, editor. Guided bone regeneration in implant dentistry. 2nd ed. Chicago, IL: Quintessence; 2009. p. 71–96.
- Rasia-dal Polo M., Poli PP., Rancitelli D., Beretta M., Maiorana C.. Alveolar ridge reconstruction with titanium meshes: a systematic review of the literature. Med Oral Patol Oral Cir Bucal. 2014 Nov;19(6):e639–46.
- Maiorana C., Santoro F., Rabagliati M., Salina S.. Evaluation of the use of iliac cancellous bone and anorganic bovine bone in the reconstruction of the atrophic maxilla with titanium mesh: a clinical and histologic investigation. Int J Oral Maxillofac Implants. 2001 May–Jun;16(3):427–32.
- Artzi Z., Dayan D., Alpern Y., Nemcovsky CE.. Vertical ridge augmentation using xenogenic material supported by a configured titanium mesh: clinicohistopathologic and histochemical study. Int J Oral Maxillofac Implants. 2003 May–Jun;18(3):440–6.
- Troeltzsch M., Troeltzsch M., Kauffmann P., Gruber R., Brockmeyer P., Moser N., Rau A., Schliephake H.. Clinical efficacy of grafting materials in alveolar ridge augmentation: a systematic review. J Craniomaxillofac Surg. 2016 Oct;44(10):1618–29.
- Sumida T., Otawa N., Kamata YU., Kamakura S., Mtsushita T., Kitagaki H., Mori S., Sasaki K., Fujibayashi S., Takemoto M., Yamaguchi A., Sohmura T., Nakamura T., Mori Y.. Custom-made titanium devices as membranes for bone augmentation in implant treatment: clinical application and the comparison with conventional titanium mesh. J Craniomaxillofac Surg. 2015 Dec;43(10):2183–8.
- Sagheb K., Schiegnitz E., Moergel M., Walter C., Al-Nawas B., Wagner W.. Clinical outcome of alveolar ridge augmentation with individualized CAD-CAM-produced titanium mesh. Int J Implant Dent. 2017 Dec;3(1):36. doi: 10.1186/ s40729-017-0097-z.
- Seiler M., Peetz M., Hartmann A., Witkowski R.. Individualized CAD/CAM-produced titanium scaffolds for alveolar bone augmentation: a retrospective analysis of dehiscence events in relation to demographic and surgical parameters. J Oral Science Rehabilitation. 2018 Mar;4(1):38–46.
- Chiapasco M., Casentini P., Tommasato G., Dellavia C., Del Fabbro M.. Customized CAD/CAM Titanium meshes for the guided bone regeneration of severe alveolar ridge defects: preliminary results of a retrospective clinical study in humans. Clin Oral Implants Res. 2021. Apr;32(4):498-510.
- Dellavia C., Canciani E., Pellegrini G., Tommasato G., Graziano D., Chiapasco M.. Histological assessment of mandibular bone tissue after guided bone regeneration with customized computer-aided design/ computer-assisted manufacture titanium mesh in humans: A cohort study. Clin Implant Dent Relat Res. 2021;1–12.
- Garocchio S., implants Italy No.2, 2021

/social-network-service/media/default/101731/b396a315.jpg)