Psychosocial profiles of painful TMD patients
SUMMARY
The aim of the present investigation is to test the null hypothesis that the presence of psychopathology in patients with temporomandibular disorders (TMD) is related to the presence of pain, independent of its location [(i.e. myofascial and ⁄ or temporomandibular joint (TMJ) pain]. Ninety-six (n = 96) patients affected by painful TMD underwent a clinical assessment in accordance with the research diagnostic criteria for TMD (RDC ⁄TMD) guidelines and filled out the Symptoms Check List – Revised (SCL-90-R) instrument to investigate the presence of symptoms of psychopathology. Patients with myofascial pain, alone or combined with TMJ pain, endorsed the highest scores in all SCL-90-R scales and showed the highest percentage of abnormal values in the depression (DEP) and somatization (SOM) scales for the assessment of depressive and somatization symptoms. Nonetheless, ANOVA revealed no significant differences between groups in any of the SCL-90-R scales, except than in the Positive Symptom Total Index (F = 3Æ463; P = 0Æ035), and the chi-squared test did not detect any significant differences between groups for the prevalence of abnormal scores in the DEP and SOM scales. The existence of a close association between pain and psychosocial disorders in TMD patients was supported by the present study. The null hypothesis is that no differences exist between patients with different painful TMD cannot be fully accepted for the presence of psychosocial disorders because of the trend evidencing higher SCL-90-R scores for myofascial pain patients, alone or combined with TMJ pain, with respect to TMJ pain alone.
INTRODUCTION
Literature data suggest that temporomandibular disorders (TMD) recognize a multifactorial aetiology in a biopsychosocial frame (1). The assessment of psychosocial disorders accompanying TMD symptoms has been the target of several recent studies (2, 3), and the diagnostic and therapeutical implications of the psychosocial impairment that derives from those disorders have been discussed in some interesting review papers (4, 5).
Several psychosocial disorders have put into relation with TMD, and literature findings support an association with anxiety (6), depression (7–9) and some personality disorders (10–12). Some earlier studies suggested that TMD patients with muscle disorders may present a higher rate of psychosocial impairment with respect to those with articular disorders (13–16).
Such findings have not been confirmed by other studies, suggesting that at least for depressive symptoms, the presence of psychosocial disorders in TMD patients seems to be related to the presence of a painful condition, and seems to be independent of the location of pain [i.e. temporomandibular joint (TMJ) or myofascial pain] (17, 18). In particular, these suggestions came from studies adopting either a peculiar spectrum approach to psychopathology (18) or some assessment items or scales that have been synthesized for use in the non-psychiatric setting (11, 17). The present investigation is an attempt to test whether if such findings were confirmed by the adoption of the full version of the Symptoms Check-List 90-R (SCL-90-R) (19), the null hypothesis for the presence of psychosocial disorders being that no significant differences exist between patients with different painful TMD.
You have the opportunity to gather more in-depth information about temporomandibular disorders diagnosis and treatment in our Online congress on evidence-based temporomandibular disorders and bruxism treatment.
MATERIALS AND METHODS
Sample size calculation
To ascertain whether the size of the study group was statistically significant, a priori calculation of the sample size necessary for this investigation was made. The values of type I and type II errors were set at 0Æ05 and 0Æ20 respectively. Data about the estimated variance were drawn from other works in the literature assuming SCL-90-R scales as the main outcome variables (11, 17).
To have an 80% statistical power to detect a statistically significant difference between groups, in consideration of the above, the needed sample size was 5–35 subjects per group.
Study design
Ninety-six (n = 96) patients affected by painful TMD were consecutively selected at the TMD Clinic, Department of Maxillofacial Surgery, University of Padova, Padova, Italy. All participants filled out the Symptoms Check List – Revised (SCL-90-R) instrument to assess psychosocial symptoms.
Clinical assessment
Subjects were clinically assessed by means of a standardized examination based on the Italian version of the research diagnostic criteria for TMD (RDC ⁄ TMD) (20). Patients were given a RDC ⁄ TMD axis I group I diagnosis of myofascial pain, with or without limited opening, if they met the following criteria:
Report of pain or ache in the jaw, temples, face, preauricular area or inside the ear at rest or during function; plus
pain reported by the subject in response to palpation of three or more of the following 20 muscles sites (right side and left side count as separate sides for each muscle): posterior temporalis, middle temporalis, anterior temporalis, origin of masseter, body of masseter, insertion of masseter, posterior mandibular region, submandibular region, lateral pterygoid area and tendon of the temporalis. At least one of the sites must be on the same side as the complaint of pain.
A RDC ⁄ TMD axis I group IIIa diagnosis of arthralgia was made according to the following criteria:
Pain in one or both joint sites (lateral pole and ⁄ or posterior attachment) during palpation; plus
one or more of the following self-reports of pain: pain in the region of the joint, pain in the joint during maximum unassisted opening, pain in the joint during assisted opening, pain in the joint during lateral excursion; plus
absence of coarse crepitus.
A RDC ⁄ TMD axis I group IIIb diagnosis of osteoarthritis was made in presence of the following criteria:
Arthralgia plus
either coarse crepitus in the joint or radiographic evidence of articular remodelling.
Patients were then divided into three groups, the first comprising subjects with a painful muscular disorder (RDC ⁄ TMD axis I group I diagnosis of myofascial pain, with or without limited opening; n = 26), the second consisting of patients with a painful articular disorder (RDC ⁄ TMD axis I group III diagnosis of arthralgia or osteoarthritis; n = 41) and the third including subjects showing both these disorders (n = 29). Patients of the study groups were included regardless of the presence of a concomitant non-painful temporomandibular disorder (i.e. disc displacement and ⁄ or osteoarthrosis).
Psychosocial assessment
All patients filled out the Italian version of the SCL-90-R for psychosocial assessment (21, 22). The SCL-90-R is widely used for self-assessment of psychological distress and multiple psychopathological dimensions. It consists of a total of 90 items, with 83 items that investigate 9 psychopathological dimensions: somatization (SOM), obsessiveness-compulsiveness (O-C), interpersonal sensitivity (I-S), depression (DEP), anxiety (ANX), hostility (HOS), phobic anxiety (PHOB), paranoid ideation (PAR) and psychoticism (PSY). In addition to these nine symptomatological dimensions, the SCL-90-R contains seven more items relating to appetite and sleep disorders. It also uses three global distress indices: the Global Severity Index (GSI), Positive Symptom Total (PST) and Positive Symptom Distress Index (PSDI) (19). We considered a cut-off score of 0Æ57 for the GSI to distinguish between a ‘functional’ and a ‘dysfunctional’ condition, according to Schauenburg and Strack (23). On the DEP subscale, scores below 0Æ535 were considered normal, between 0Æ535 and 1Æ105 indicated moderate DEP and above 1Æ105 the presence of severe ongoing depressive disorder. On the SOM subscale, including the pain items, scores lower than 0Æ5 were considered normal, values between 0Æ5 and 1 indicated moderate SOM and above 1 severe SOM (3).
Statistical analysis
ANOVA followed by Bonferroni’s test correction was used to compare mean values obtained by the three groups of TMD patients on various SCL-90-R scales. Chi-squared test was performed to compare the prevalence of patients scoring over or under cut-off values for DEP and SOM scales. Statistical significance was set at P < 0Æ05. All statistical analyses were performed with the Statistical Package for the Social Sciences 11Æ0.*
RESULTS
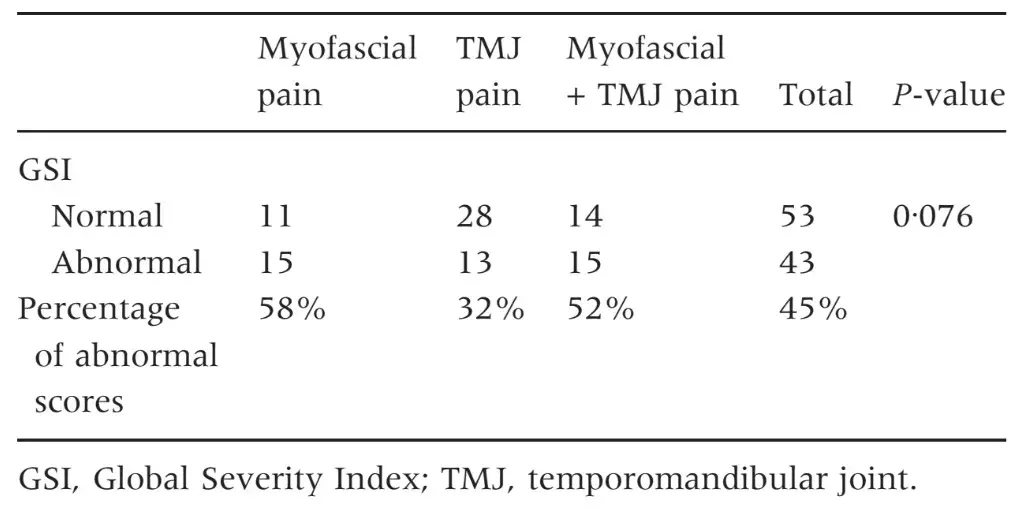
The three groups of patients were not different for mean age and female:male ratio (Table 1) as well as for the prevalence of persistent pain from more than 6 months (Table 2). Patients with myofascial pain, alone or combined with TMJ pain, endorsed the highest scores in almost all SCL-90-R scales. ANOVA showed that differences between groups were not significant at P = 0Æ05 value in any of the SCL-90-R scales except in PST Index (F = 3Æ463; P = 0Æ035) (Table 3). As for the prevalence of abnormal values in the SOM and DEP scales, myofascial pain patients’ groups were those showing the highest percentage of abnormal values, even though chi-squared analysis did not detect any significant differences between groups (Tables 4 and 5). Similar findings were showed for the GSI as well (Table 6).
Table 1. Mean age and sex ratio of the three groups of patients
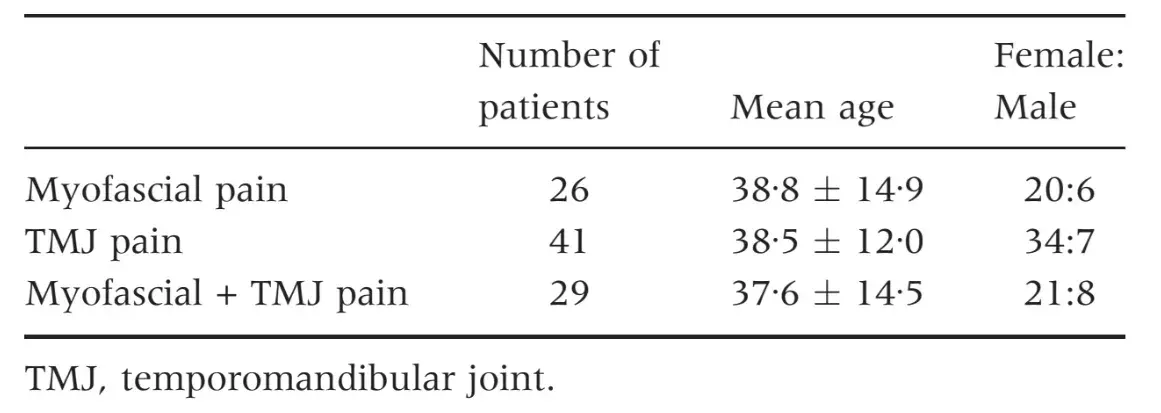
Table 2. Prevalence of persistent pain (lasting from more than 6 months) in the TMD groups
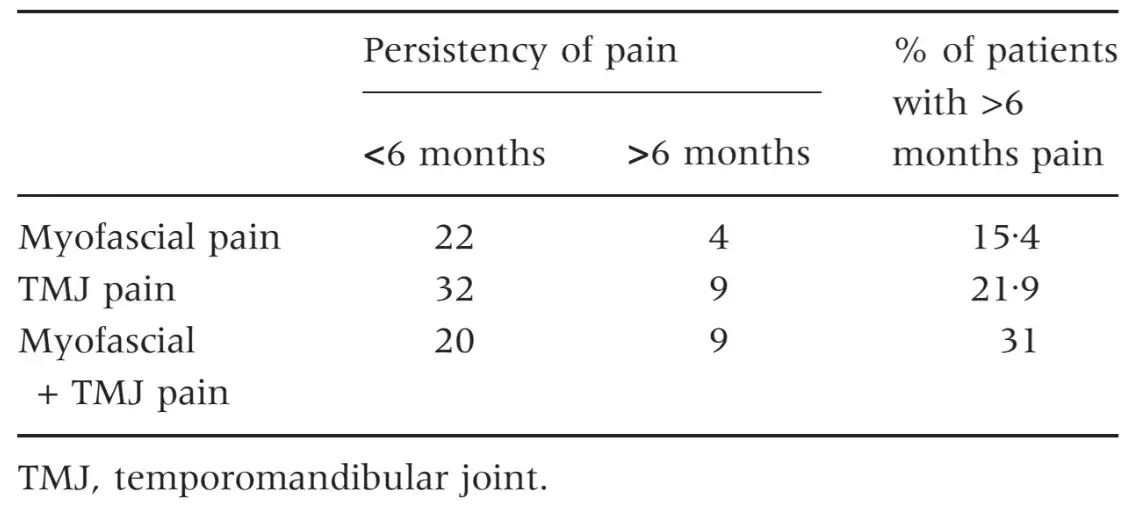
Table 3. Mean values of the three groups of patients in the SCL-90-R scales and results of ANOVA test
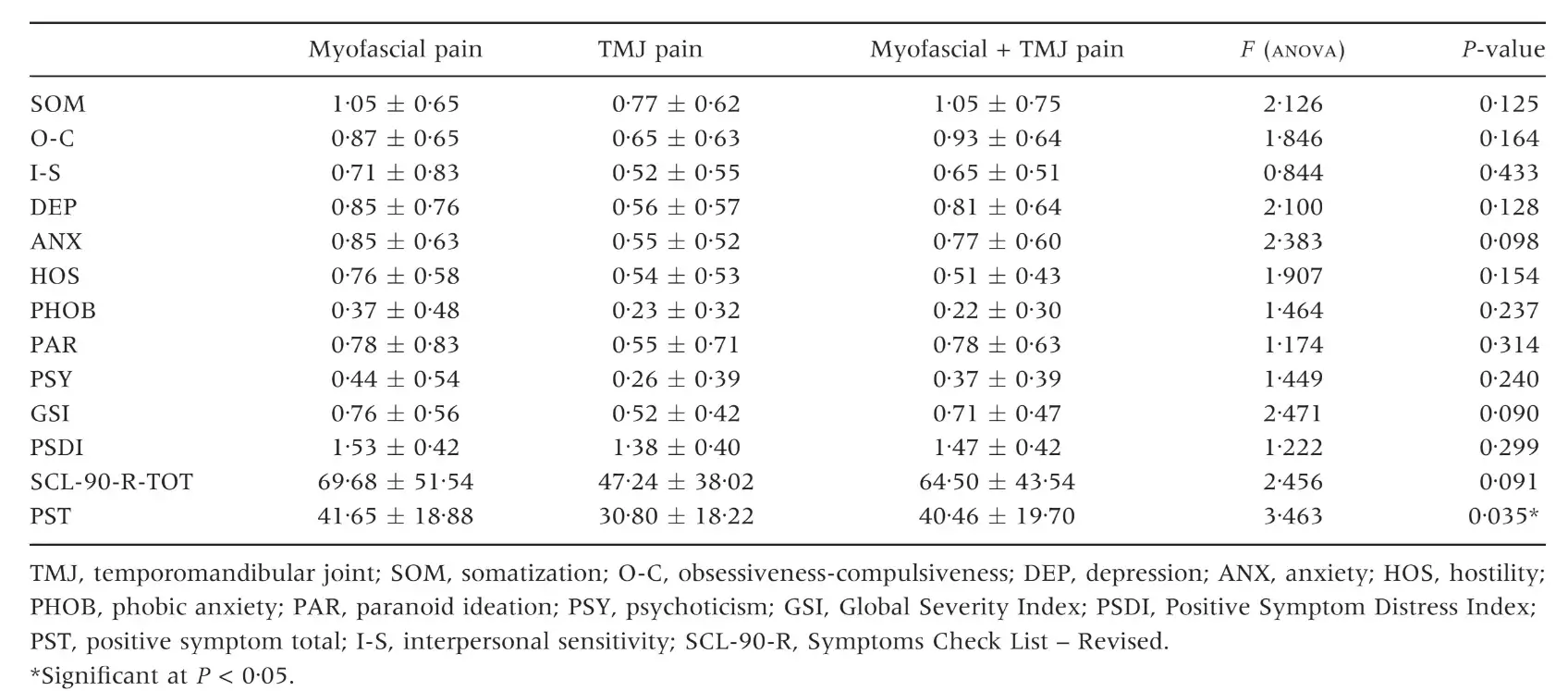
Table 4. Number of patients with normal, moderate or severe levels of somatization (SOM scale) and percentage of patients with abnormal scores (moderate + severe)
Table 5. Number of patients with normal, moderate or severe levels of depression (DEP scale) and percentage of patients with abnormal scores (moderate + severe)
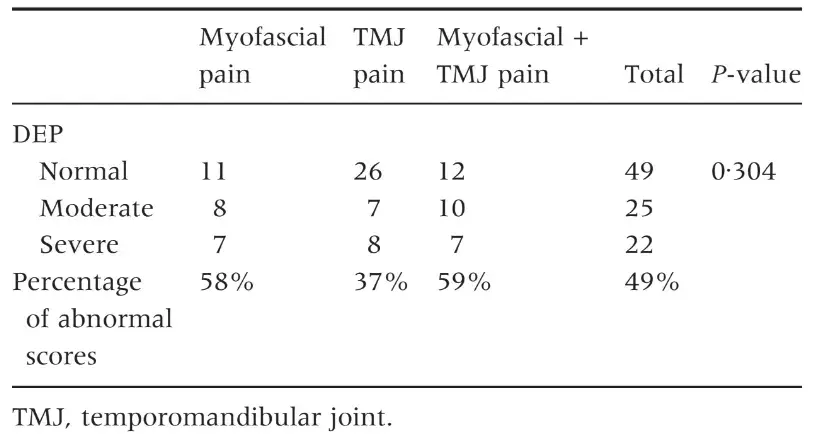
Table 6. Number of patients and percentage of abnormal scores in the GSI scale
DISCUSSION
Literature data on TMD clearly support the existence of an association with a number of psychosocial disorders, such as ANX, DEP and SOM disorders (4, 10, 24–28). Despite abundant works on this particular issue, paucity of data exist about the prevalence of those disorders in the different TMD forms. In particular, while some early works suggested that populations of patients with muscular TMD forms were the most compromised from a psychosocial viewpoint (13–16), there is an increasing evidence that the complex relationship between psychopathology and TMD could actually depend upon the presence of painful TMD conditions and not upon the location of the disorder (11, 18, 29).
Such suggestion came mainly from a single study adopting a peculiar spectrum approach to psychopathology (18) and was in line with observations from a series of studies on Asian TMD patients adopting a more traditional approach to psychopathology assessment such as the adoption of the SCL-90-R scales that have been included in the RDC ⁄ TMD axis II (11, 18, 30, 31). More recently, the hypothesis that the location of pain is not a major factor in the prediction of psychosocial profiles of TMD patients found support in a work by Reissmann et al. (29) who compared the Multidimensional Pain Inventory (MPI) and RDC ⁄ TMD axis II profiles of TMD patients with different pain location and found no significant differences between groups. In the present investigation, three groups of patients with different painful TMD filled out the full version of the SCL-90-R instrument to work on the hypothesis that no difference in their psychometric profile should be found.
Patients with myofascial pain, alone or combined with TMJ pain, endorsed the highest scores in all the SCL-90-R scales, but differences with patients with TMJ pain alone were not significant in any of the instrument’s scale. Such findings are comparable with data from an early similar work by Yap et al. (11) who found that myofascial pain and TMJ pain patients were the most compromised from a psychosocial viewpoint.
In particular, they focused their analysis on the SCL-90-R scales for the assessment of DEP and SOM. Differences with results from the present investigation are of minor importance, if one consider that DEP scores of the three groups covered a 0Æ57–0Æ98 range versus the 0Æ56–0Æ85 range in the present study, and SOM scores were within a 0Æ58–1Æ07 range versus 0Æ77–1Æ05 range.
The present findings on the percentage of patients with above-threshold scores in the DEP scale (49% versus 52%) are also comparable to those of Yap et al. (11), while minor differences emerged with respect to the SOM scale. Also from findings of Reissmann et al. (29), even reporting lower values of DEP and SOM prevalence confirmed a close association between TMD and those two psychosocial disorders. Such results have been further reported in a series of papers by Yap et al. (18, 31) who concluded that DEP and SOM are related to the portion of the RDC ⁄ TMD axis I clinical examination that requires self-reporting of pain. Thus, literature findings seem to point out that the widely described TMD-psychosocial disorders association may be part of the more complex pain-psychopathology association, at least for symptoms of the DEP and SOM spheres.
The present investigation, by the use of the full version of the SCL-90-R instrument, allowed extending the same considerations to the other aspects of the psychosocial sphere that are investigated by such instrument, with particular regard to ANX and PHOB symptoms, which, by contrast, were supposedly associated to myofascial pain rather than TMJ disorders and were never been addressed before with a comprehensive evaluation. Nonetheless, the independence of the pain-psychosocial disorders association with regards to pain location cannot be considered definitive for several reasons, the first of which being the small number of researchers involved and the peculiarity of instruments adopted in some studies.
Despite the absence of statistically significant differences between groups, it must be remembered that myofascial pain patients’ scores have been generally higher than those patients with TMJ pain alone in the present investigation as well as in those by Yap et al. (11, 17) and at least for SOM scores, Reissmann et al. (29). The level of psychosocial distress in myofascial pain patients did not increase with the presence of TMJ pain as scores of patients with myofascial pain alone or combined with TMJ pain are very similar. These common findings are hardly because of chance, and there is a strong need to get deeper into their analysis in the near future.
In particular, generalization of the present findings is not possible due to some potential bias related to the presence of a low percentage of patients with chronic painful symptoms, which is not representative of TMD patients as a whole. Indeed, the inclusion of a higher number of chronic TMD patients might provide different results with respect to the present findings and should be considered in the design phase of future studies.
Moreover, the power analysis to assess the needed sample size for this investigation was based on previous studies’ findings to estimate the expected variance of SCL-90-R scores, but the validation for differences in scoring ranges could not be provided due to the absence of comparable literature data on the interpretation of SCL-90-R scores. Thus, studies on larger and more representative populations of TMD patients are strongly needed.
As for the interpretation of results in terms of a potential causal link between painful TMD and psychosocial disorders, the cross-sectional design of the investigation did not allow drawing conclusions as to whether the psychosocial disorders described in the present populations of TMD patients are a consequence of the clinical symptoms or the expression of an underlying personological risk factor for TMD.
Obviously, a discussion of the causal link between pain and depression is beyond the scope of this investigation, and it should be addressed with appropriately designed studies by taking into account the pain chronicity as the main study’s variable. Taking all considerations together, it appears that there is a need for more basic research studies that allows to detect possibly different neurophysiological and ⁄ or endocrinological pathways which are responsible for the patients’ coping with myofascial and TMJ pain.
There are additional information about TMJ pathology treatment in our course "Evidence-based treatment of TMJ dysfunction".
CONCLUSIONS
Findings from the present investigation supported the existence of a close association between pain and psychosocial impairment in TMD patients, also suggesting that the presence of psychosocial distress may be independent by the location of pain, at least for the subtypes of TMD studied. Nonetheless, some potential sampling bias suggests that there are not enough elements to fully accept the null hypothesis that no differences exist between patients with different painful TMD for the presence of psychosocial disorders. Besides, some minor differences exist between patients with myofascial pain, alone or combined with TMJ pain, and those with TMJ pain alone. Future studies are encouraged to address possible pathophysiological mechanisms underlying these differences to verify their actual importance, if existing, in the clinical setting.
References
Greene C. The etiology of temporomandibular disorders: implications for treatment. J Orofac Pain. 2001;15:93–105.
Turner JA, Dworkin SF, Mancl L, Huggins K, Truelove E. The roles of beliefs, catastrophizing, and coping in the functioning of patients with temporomandibular disorders. Pain. 2001;92:41–51.
Dworkin SF, Sherman J, Mancl L, Ohrbach R, LeResche L, Truelove E. Reliability, validity, and clinical utility of the research diagnostic criteria for temporomandibular disorders axis II scales: depression, non-specific physical symptoms, and graded chronic pain. J Orofac Pain. 2002;16:207–220.
Rollman GB, Gillespie JM. The role of psychosocial factors in temporomandibular disorders. Curr Rev Pain. 2000;4:71–81.
Suvinen TI, Reade PC, Kemppainen P, Kononen M, Dworkin SF. Review of aetiological concepts of temporomandibular pain disorders: toward a biopsychosocial model for integration of physical disorders with psychological and psychosocial illness impact factors. Eur J Pain. 2005;9:613–633.
Madland G, Feinman C, Newman S. Factors associated with anxiety and depression in facial arthromyalgia. Pain. 2000;84:225–232.
Gatchel RJ, Garofalo JP, Ellis E, Holt H. Major psychological disorders in acute and chronic TMD: an initial examination. J Am Dent Assoc. 1996;127:1365–1374.
Auerbach S, Laskin D, Frantseve LME, Orr T. Depression, pain, exposure to stressful life events and long term outcomes in temporomandibular disorder patients. J Oral Maxill Surg. 2001;59:628–633.
Manfredini D, Bandettini di Poggio A, Romagnoli M, Dell’Osso L, Bosco M. A spectrum approach for the assessment of manic-depressive symptoms accompanying temporomandibular disorders. Minerva Stomatol. 2003;52:231–240.
Mongini F, Ciccone J, Ibertis F, Negro C. Personality characteristics and accompanying symptoms in temporomanibular joint dysfunctions, headache and facial pain. J Orofac Pain. 2000;14:52–58.
Yap AUJ, Dworkin SF, Chua EK, List T, Tan KBC, Tan HH. Prevalence of temporomandibular disorders subtypes, psychologic distress and psychosocial dysfunction in asian patients. J Orofac Pain. 2003;17:21–28.
Nifosı` F, Violato E, Sifari L, Novello G, Guarda-Nardini L, Manfredini D et al. Clinical psychiatric assessment and psychopathological measure of patients with myofascial and temporomandibular joint pain. Int J Psychiatry Med. 2007; 37:283–300.
Harness D, Donlon W, Eversole L. Comparison of clinical characteristics in myogenic, TMJ internal derangement and atypical facial pain patients. Clin J Pain. 1990;6:4–17.
Michelotti A, Martina R, Russo M, Romeo R. Personality characteristics of temporomandibular disorder patients using MMPI. Cranio. 1998;16:119–125.
Kight M, Gatchel RJ, Wesley L. Temporomandibular disorders: evidence for significant overlap with psychopathology. Health Psychol. 1999;18:177–182.
Manfredini D, Bandettini Di Poggio A, Cantini E, Dell’Osso L, Bosco M. Mood and anxiety psychopathology and temporomandibular disorders: a spectrum approach. J Oral Rehabil. 2004;31:933–940.
Yap AUJ, Tan KBC, Chua EK, Tan HH. Depression and somatization in patients with temporomandibular disorders. J Prosthet Dent. 2002;88:479–484.
Manfredini D, Bandettini di Poggio A, Romagnoli M, Dell’Osso L, Bosco M. Mood spectrum in patients with different painful temporomandibular disorders. Cranio. 2004;22:234–240.
Derogatis LR. SCL-90-R. Administration, scoring and procedures. Manual – II. Towson, MD: Clinical Psychometric Research; 1983.
Dworkin S, LeResche L. Research diagnostic criteria for temporomandibular disorders: review, criteria examinations and specifications, critique. J Craniomandib Disord Fac Oral Pain. 1992;6:301–355.
Derogatis LR, Lipman RS, Covi L. SCL-90: an outpatient psychiatric rating scale. Preliminary report. Psychopharmacol Bull. 1973;9:13–28.
Conti L. Repertorio delle scale di valutazione in psichiatria (Italian). Pisa, Italy: SEE Ed, 1999.
Schauenburg H, Strack M. Measuring psychotherapeutic change with the symptom checklist SCL 90 R. Psychother Psychosom. 1999;68:199–206.
Manfredini D, Landi N, Bandettini di Poggio A, Dell’Osso L, Bosco M. A critical review on the importance of psychological factors in temporomandibular disorders. Minerva Stomatol. 2003;52:321–330.
Gallagher RM, Marbach JJ, Raphael KG, Dohrenwend BP, Cloitre M. Is major depression comorbid with temporomandibular pain and dysfunction syndrome? A pilot study Clin J Pain. 1991;7:219–225.
Sirirungrojying S, Srisintorn S, Akkayanont P. Psychometric profiles of temporomandibular disorder patients in southern Thailand. J Oral Rehabil. 1998;25:541–544.
Meldolesi GN, Picardi A, Accivile E, Toraldo di Francia R, Biondi M. Personality and psychopathology in patients with temporomandibular joint pain-dysfunction syndrome. Psychother Psychosom. 2000;69:322–328.
Sipila K, Vejola J, Jokelainen J, Jarvelin MR, Oikarinen K, Raustia A et al. Association between symptoms of temporomandibular disorders and depression: an epidemiological study of the Northern Finland 1966 birth cohort. Cranio. 2001;19:183–187.
Reissmann DR, John MT, Wassell RW, Hinz A. Psychosocial profiles of diagnostic subgroups of temporomandibular disorder patients. Eur J Oral Sci. 2008;116:237–244.
Yap AUJ, Chua E, Hoe KE. Clinical TMD, pain-related disability and psychological status of TMD patients. J Oral Rehabil. 2002;29:374–380.
Yap AUJ, Chua EK, Tan KBC. Depressive symptoms in Asian TMD patients and their association with non-specific physical symptoms reporting. J Oral Pathol Med. 2004;33:305–310.


